What company makes belimumab?
GlaxoSmithKline plc (LSE/NYSE: GSK) announced the US Food and Drug Administration (FDA) has approved BENLYSTA (belimumab) for the treatment of adult patients with active lupus nephritis (LN) who are receiving standard therapy.
How much does belimumab cost?
Belimumab is available in 120-mg and 400-mg vials with approximate costs of $443 and $1,477, respectively; thus, a weight-based dosing regimen costs approximately $35,000 per year.
Who manufactures BENLYSTA?
GSK announced today that the US Food and Drug Administration (FDA) has approved a new subcutaneous formulation of Benlysta (belimumab) for the treatment of adult patients with active, autoantibody‑positive SLE who are receiving standard therapy.
Who sells BENLYSTA?
1. Specialty Distributor:
- ASD Specialty Healthcare 1-800-746-6273.
- Besse Medical 1-877-728-3476.
- Cardinal Health Specialty 1-866-476-1340.
- CuraScript 1-877-599-7748.
- McKesson Specialty 1-800-482-6700.
- McKesson Plasma and Biologics 1-877-625-2566.
- Metro Medical 1-800-768-2002.
- Oncology Supply 1-800-633-7555.
Is belimumab a biologic?
The drug, belimumab, which was approved on March 9, 2011, by FDA, is the first ever targeted biological for the treatment of SLE patients with active, autoantibody-positive disease, who are already on standard therapy. [5] It has been developed by Human Genome Sciences Inc.
Is belimumab a chemo drug?
No, Benlysta isn’t a chemotherapy drug. It’s a type of drug called a monoclonal antibody (a biologic drug made from immune system cells). People with lupus have an immune system that attacks their body. Benlysta works by weakening your immune system, which can help relieve your lupus symptoms.
Is belimumab expensive?
Benlysta (belimumab) is a member of the selective immunosuppressants drug class and is commonly used for Lupus Nephritis, and Systemic Lupus Erythematosus….Intravenous Powder For Injection.
| Quantity | Per unit | Price |
|---|---|---|
| 1 | $592.32 | $592.32 |
How is belimumab administered?
BENLYSTA may be administered as an intravenous infusion or as a subcutaneous injection. Vials are intended for intravenous use only (not for subcutaneous use) and autoinjectors and prefilled syringes are intended for subcutaneous use only (not for intravenous use).
Is belimumab a steroid?
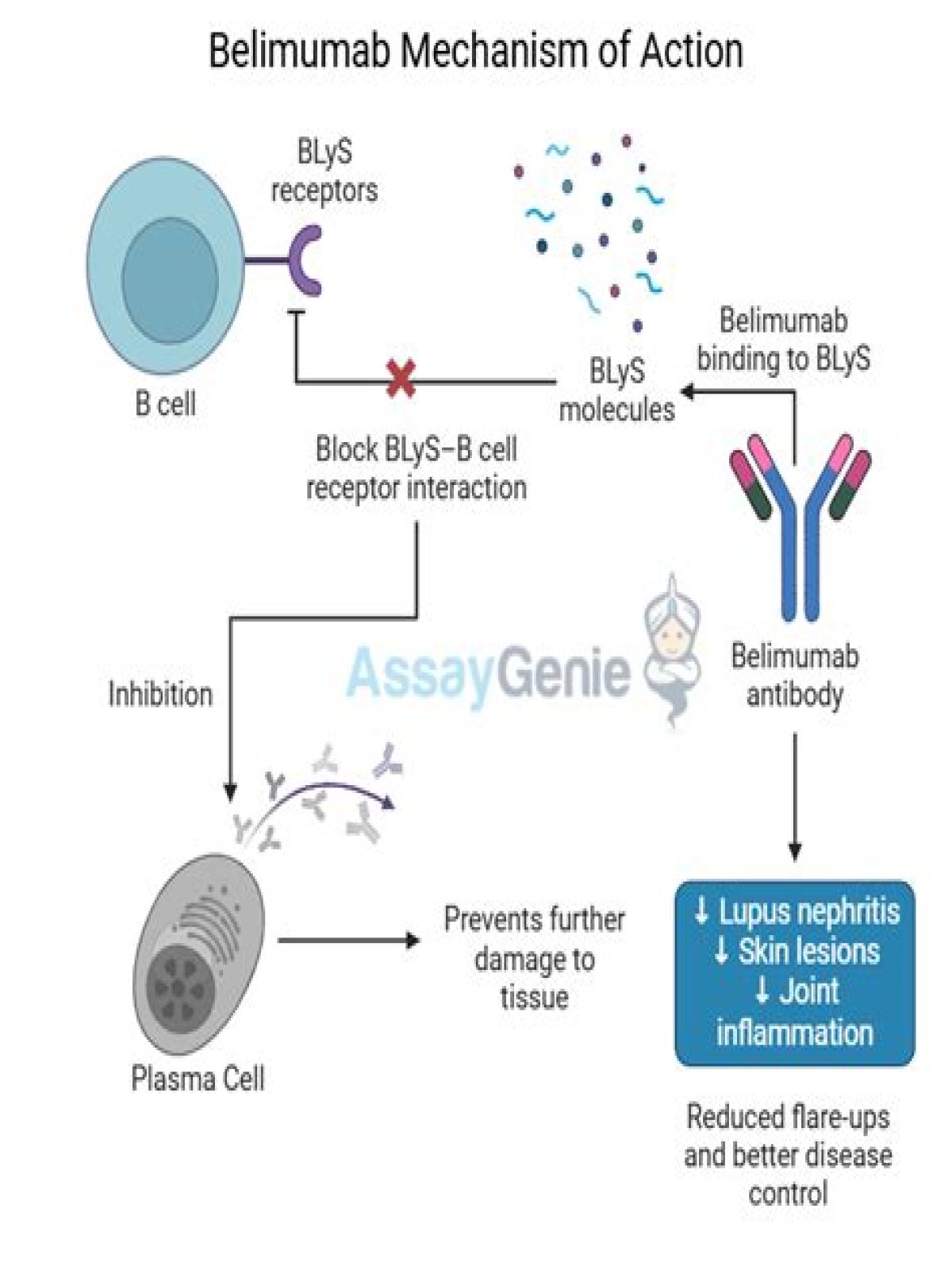
BENLYSTA is a biologic therapybiologic therapy: A treatment derived from living tissues or cells., not a steroidsteroid: Treatment to reduce the swelling, pain, and other symptoms of inflammation.. It’s the only FDA-approved treatment that targets BLySBLyS: BENLYSTA works by binding to BLyS.
What are the side effects of belimumab?
Common side effects of Benlysta include:
- nausea,
- vomiting,
- diarrhea,
- stomach pain,
- pain in your arms or legs,
- trouble sleeping (insomnia),
- headache (migraine),
- fever,
What is the life expectancy of someone with lupus?
With close follow-up and treatment, 80-90% of people with lupus can expect to live a normal life span. It is true that medical science has not yet developed a method for curing lupus, and some people do die from the disease. However, for the majority of people living with the disease today, it will not be fatal.
When was belimumab FDA approved?
Belimumab Approved In late December 2020, the U.S. Food & Drug Administration (FDA) approved belimumab (Benlysta) to treat adults with active lupus nephritis who are receiving standard therapy.
Is belimumab a steroid or a biologic?
BENLYSTA (belimumab) is a biologic therapy biologic therapy: A treatment derived from living tissues or cells., not a steroid steroid: Treatment to reduce the swelling, pain, and other symptoms of inflammation..
What is the FDA approval for belimumab for the treatment of SLE?
Under the trade name Benlysta, belimumab received FDA approval for the treatment of SLE on March 9, 2011, despite concerns among advisory committee members that the improvement of 4 points on the SELENA-SLEDA scale was marginal, and despite reservations about additional deaths in the treatment group.
How much does belimumab cost in the US?
At a typical U.S. academic center, the total cost for the first year of treatment with belimumab is $28,000. Belimumab is much more expensive than other drugs used to treat lupus, including prednisone ($140 per year), hydroxychloroquine ($132), oral methotrexate ($432), azathioprine ($468), and mycophenolate mofetil ($1,224).
What type of antibody is belbelimumab?
Belimumab is a human IgG1 neutralizing monoclonal antibody against B-lymphocyte stimulating factor (also known as B-lymphocyte stimulator [BLyS]).50 BLyS, a member of the tumor necrosis factor (TNF) ligand superfamily, is synthesized as a 285-amino acid type II membrane protein and exists in both membrane and cleaved 152-amino acid soluble forms.